Reports: AC2
44141-AC2 The Development of the Sulfur Isotopic Composition of Carbonate Associated Sulfate as an Indicator of Diagenetic Environment in the Formation of Dolomite
Dolomitized reservoirs are currently responsible for a large percentage of the world's oil and gas reserves. However, in spite of the economic significance of these fields and the importance of dolomitization in altering reservoir characteristics, the mechanism of dolomite formation is a matter of speculation. The work proposed in this grant aims to understand the using of the sulfur isotopic composition d34S of carbonate associated sulfate (CAS) trapped within the dolomite as an indicator of the environment of dolomitization.
During this grant we performed the following work: (i) established a method for removing contamination from dolomite phases, (ii) constructed a modified Dumas combustion system interfaced to an Europa 20-20 mass spectrometer for the analysis of the d34S of SO2 produced from the oxidation of BaSO4; (iii) measured the sulfur isotopic composition from bulk carbonates and separated dolomites from Site 1129 of the ODP (Figure 1), a core from San Salvador in the Bahamas, and dolomites from the Madison formation in Wyoming. Publications for GCA and Sedimentology are in preparation.
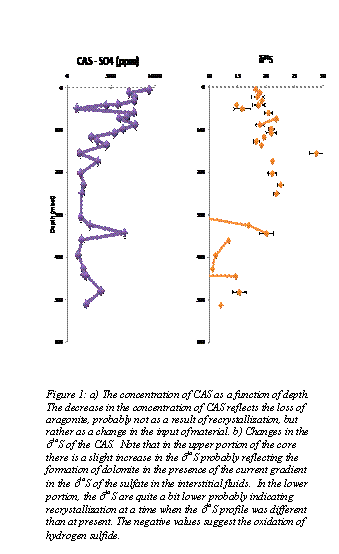
The issue of carbonate associated sulfate (CAS), or sulfur trapped within the matrix of carbonate minerals, has attracted a significant deal of attention because of the possibility of utilizing this source of sulfur to refine the oceanic sulfur isotopic curve which at present is based mainly on the analysis of evaporite minerals. In addition to obtaining the d34S of the original depositional seawater, the d34S can provide information in conjunction with the concentration of non-conventional trace elements (S, Na, K, and Cl) regarding the nature of the environment of diagenesis. For example, it is well known that many dolomites are formed within the sulfate reduction zone, where dolomitization is promoted by the degradation of organic material (creating alkalinity) and perhaps by the removal of the inhibitory sulfate ion. Such dolomite would have lower concentrations of S, normal concentrations of Na, K, and Cl, but slightly elevated d34S values. In this environment the concentration of sulfate would be expected to be lower than in normal marine sediment and the d34S would be slightly enriched as the process of sulfate reduction forms H2S depleted in d34S thereby enriching the residual sulfate. Dolomites associated with evaporite minerals might have low concentrations of SO42- (as SO42- is removed during the formation of evaporite minerals), normal d34S values, but elevated values of Na+, K+, and Cl-. Dolomites formed from brines which have not attained saturation with respect to gypsum or anhydrite might be expected to show elevated concentrations of all non-conventional trace elements including sulfate and normal d34S values. Preliminary results from Site 1129, cored during Leg 182 of the ODP are shown in Figure 1. The results of this study show that in the upper portion of the profile show a slight increase in the d34S . This increase in much less than the increase in the d34S of the dissolved sulfate (data from Wortmann et al., unpublished), but the increase is consistent with the precipitation of about 5-10% dolomite.
In the lower portion of the core, the d34S values of the bulk sediment are quite low (~10) and suggest the oxidation of isotopically light H2S to sulfate and then subsequent incorporation into the diagenetic carbonate. As there are no intervals in the present porewater profile which show this pattern, this must have taken place during a earlier period when the porewater profile was different than today. The data indicate that (i) the dolomite in the lower portion of the core is not forming at the present time, (ii) dolomite in the upper portion of the core is forming under the present geochemical regime, (iii) at times in the past oxidation of hydrogen sulfide has contributed to the sulfate pool.
Figure 1: a) The concentration of CAS as a function of depth. The decrease in the concentration of CAS reflects the loss of aragonite, probably not as a result of recrystallization, but rather as a change in the input of material. b) Changes in the d34S of the CAS. Note that in the upper portion of the core there is a slight increase in the d34S probably reflecting the formation of dolomite in the presence of the current gradient in the d34S of the sulfate in the interstitial fluids. In the lower portion, the d34S are quite a bit lower probably indicating recrystallization at a time when the d34S profile was different than at present. The negative values suggest the oxidation of hydrogen sulfide.