Reports: AC6
48149-AC6 Mass Spectrometric Studies of Hydrophobic Interactions in Binary Clusters
The sections below describe results of experimental studies carried out at Purdue in the past year with ACS-PRF support.
Binding Energies in Hydrophobic Clusters
In the last year, we received funding from the ACS-PRF and a Purdue Research Foundation fellowship to investigate the binding in hydrophobic clusters. The types of systems to be investigated include binary clusters between aromatic molecules and water.
We have recently shown that the binding energies in neutral clusters can be measured by using ion chemistry and mass spectrometry. A thermochemical cycle that illustrates our approach is shown in Figure 3. The binding enthalpy of the neutral cluster (BDE(A-B)) is related to the proton affinities of the cluster (PA(A-B)) and the monomer (PA(A)) and the solvation energy of the ion by A (SE(BH+-A)) according to equation 1. Simple proton affinities are generally well-known, and
BDE(A-B) = PA(B) + SE(BH+-A) PA(A-B) (1)
solvation energies are often known or can be measured. The most challenging step is the determination of the dimer proton affinities, which can be obtained by mass spectrometric methods. Thus, in our recent study of the water dimer, the hydrogen bond energy was obtained from the proton affinities of water and water dimer, and the bond energy between hydronium ion and water. Our measured value, 18 ± 9 kJ/mol, is in good agreement with other experimental and theoretical results, showing that the approach can be used successfully to measure BDEs in weakly bonded clusters.
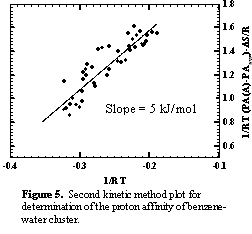
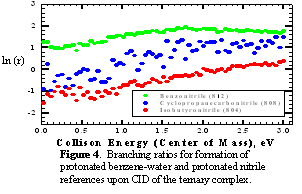
Studies carried out in the initial phase of the project have focused on the determination of the bond energy of the benzene-water cluster, B-W. Zwier and co-workers have determined by using spectroscopic measurements that the binding energy is between 7 and 12 kJ/mol, and the binding energy has been measured by using photoionization spectroscopy to be 10 kJ/mol. We have now measured the binding energy by using our ion chemistry approach. Preliminary results of this work have been presented by graduate student Laura Haupert in a Physical Chemistry seminar in March, and are currently being written for publication. The proton affinity of benzene-water cluster has been measured by using the kinetic method. Branching ratios for formation of protonated B-W and protonated reference upon CID of protonated ternary clusters of benzene, water, and nitrile reference are shown in Figure 4. The second kinetic method plot is shown in Figure 5. The measured proton affinity is obtained by adding the slope of the plot in Figure 5 to the average proton affinities of the references, 808 kJ/mol, giving a value of PA(B-W) of 813 kJ/mol. By using the thermochemical cycle shown in Figure 3, the B-W binding energy can be calculated by using eq 1.
Using the measured PA in this work, and values of SE(HW+-B) and PA(B) from the literature, the binding energy in benzene-water is found to be 8 kJ/mol, in excellent agreement with the previously reported values.
The experiments described above have established that the ion chemistry approach can be used successfully to measure binding energies in weakly bound, hydrophobic clusters. This was the only the first step in the project, but is essential in order to proceed. With the viability of the methodology established, we are now beginning to address other types of systems that are not as well known. The most important advantage of the mass spectrometric approach is its flexibility. It is readily amenable to other aromatic substrates, such as toluene, halogenated benzenes, aniline, pyridine, and phenol. Of these systems, only the hydration energies of protonated aniline and protonated pyridine are known, and so solvation energies will have to be measured. These can be determined by using energy-resolved CID of the binary cluster ions. Our current work is focusing on clusters of chlorobenzene and water to investigate the role of polarity on the cluster binding.