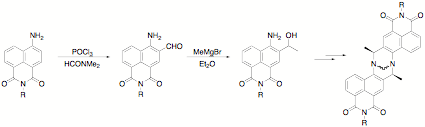Reports: B1
44989-B1 Stereochemical Studies of Fluorescent Troger's Bases
Scientific Progress:
The proposed approach to the synthesis of the monomeric precursors of the required fluorescent Trger's bases required sequential formylation of an N-alkyl-4-amino-1,8-naphthalimide, Grignard addition to the aldehyde, and optical resolution of the resultant secondary alcohol.
This project has been a source of considerable frustration due to the failure of key reactions to occur, or due to the difficulty we have had in reproducing reactions. This has been all the more surprising given our long acquaintance with this ring system and its reactions.
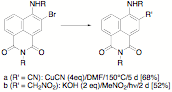
Electrophilic substitution at the 3-position of the ring has proved to be quite facile by bromine and concentrated nitric acid, but our experience following the initial discovery that we could couple the 3-bromo derivative with copper (I) cyanide under Ullmann conditions has proved to be less reproducible than we had originally found; the first student had relatively little problem duplicating the reaction (although with gradually decreasing yields), but subsequent students attempting to duplicate the reaction have found that the reaction fails more often than not. The same has proven true with the Nef reaction, displacing the bromine with the anion of nitromethane. Again, while our initial results were positive, their reproduction has proved to be problematical. Anticipatimng that the nitro group might be a better leaving group under conditions of nucleophilic aromatic substitution, we attempted the displacement of the 3-nitro group by cyanide anion, but were unsuccessful in displacing the nitro group, which appears to increase the acidity of the alkylamino group to the point where its deprotonation is favored over addition of the nucleophile to the ring.
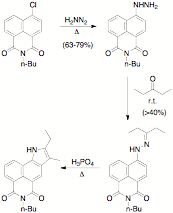
This has led to a complete re-evaluation of our strategy this past year, and we are now making slow progress using the annulated pyrrolo derivative as an entry to the acylated aminonaphthalimide that we need. We have successfully converted the chloronaphthalimide into the corresponding hydrazinonapththalimide, and this reacts with 3-pentanone to give the corresponding hydrazone in adequate yield after recrystallization. The Fischer indole synthesis based on this hydrazone is also being problematic, but we are confident that we will be able to overcome the problems we are currently encountering. NMR analysis of the reaction kxitures is showing that we are, in fact, forming the required indole, but the desired compound is still accompanied by a large number of by-products that we have not yet been able to separate. The optimization of the reaction is now under active study. With the indole in hand, we propose to oxidize the indole to give the 3-acetyl-4-propionylamino derivative, which we are confident we will be able to elaborate to the required Trger's base (although our experience in this system otherwise does render this prediction potentially hollow).
The reasons for using this circuitous route to the 3-acyl compound is simple—we have been unable to effect direct ring acylation of the simple amino compound, nor of any alkylamino compound. In every case that we have studied, the major product has been the 4-acylamino compound rather than the desired 3-acyl-4-amino-1,8-naphthalimide derivative.
Impact on the P.I. and students
The grant has provided much needed support to move our work in the area of fluorescent compounds forward. After the discoveries of new naphthalimide reactivity last year, the project has become significantly more problematic. We are following several new leads for constructing the dibenzodiazocine ring system. It is important at this stage to get our observations of heretofore unreported reactivity in the naphthalimide system into publishable form, and to publish these results. We expect this to be a fertile area of research for the future, and to extend this line of research beyond the end of this grant.
Three students have been directly involved in this project for some period during the reporting period. Kelsey Dunkle graduated with a B.S. degree in chemistry, and is pursuing graduate study at North Dakota State University; I had thought that her work completing the study of the abnormal bromination of the N-allyl systems had that project ready for publication, but several details remain to be cleaned up before the manuscript is ready for submission. I anticipate that this will occur during the between-semester break this year. Elizabeth Raupach has now graduated, and is now in graduate school in molecular biology at the University of Pittsburgh. Her work on the Ullmann and Nef reactions will be published once we have found the reasons for the lack of reproducibility of the reaction in our hands. Kyle Kopidlansky has moved onto the project full-time and is completing the work of Leah Groess for publication. This work is in the final stages, and the manuscript will be submitted before the end of this semester.
Brad Klemm joined the research group in September, and will be supported on the project if a no additional funds extension is approved. He will, however, continue on the project regardless of support, and he is proving to be an excellent practical chemist.