Reports: AC7
45823-AC7 Highly Functionalized Macromolecules Based on Free Radical Copolymerization of Substituted Stilbene Monomers
Functionalized stilbenes are a group of versatile monomers used as electron donors for free radical copolymerization with electron acceptors, like maleic anhydride and substituted N-phenylmaleimides. The pendant phenyl rings from stilbenes can impart potential rigidity into the polymer backbone. In addition the rich chemistry available for stilbenes makes the preparation of a variety of functional stilbene derivatives possible, which will provide interesting properties and applications for the corresponding polymers. Our research aims to synthesize and characterize a novel group of rod-like polymers incorporating functionalized stilbene units and then explore the unique properties and applications of these polymers. Currently there are two specific projects underway: free radical copolymerization of methyl substituted stilbenes with maleic anhydride and synthesis and characterization of rigid polyelectrolytes with different charges and charge densities.
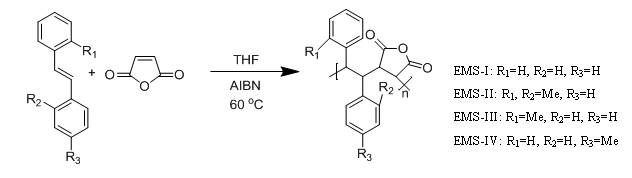
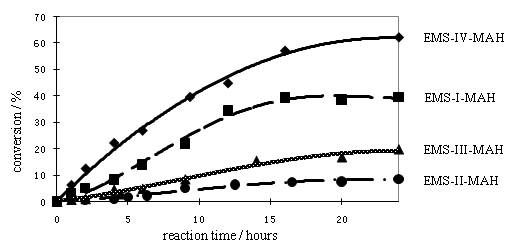
In the first project, our objective is to investigate the impact of the different locations of the methyl substituent on the reactivity of stilbenes and the corresponding copolymerization rates with maleic anhydride (MAH). We prepared several methyl substituted (E)-stilbene (EMS) monomers and copolymerized them with maleic anhydride (Scheme 1). We observed significant difference in the rates of polymerization with maleic anhydride (Figure 1), which indicates that methyl substituent on the phenyl ring of stilbene can change the reactivity of stilbene by changing the resonance stability of the propagating radical and steric hindrance in the propagation step. (1)
Figure 1
We also observed the significant difference in the polymer molecular weights depending on the position of the methyl groups on the phenyl ring. Size exclusion chromatography (SEC) traces for poly((E)-4-methylstilbene-alt-maleic anhydride) exhibited bimodal peaks. Dynamic light scattering (DLS) analysis confirmed the formation of interchain aggregates. We are investigating the origin and scope of this aggregation phenomenon in the stilbene system.
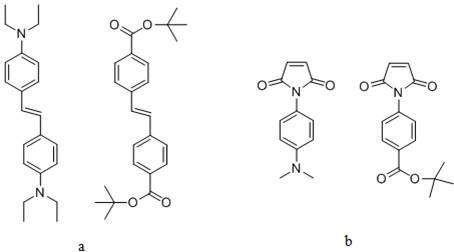
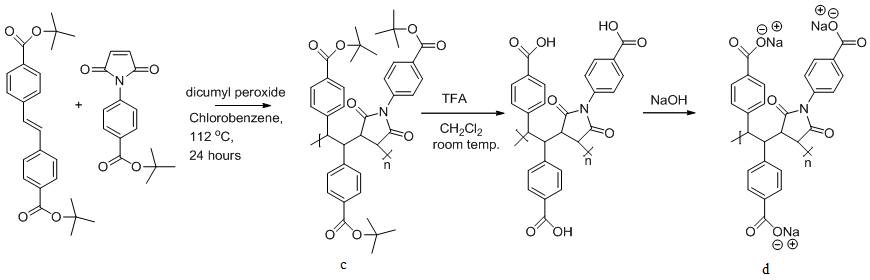
In the second project, we have prepared numerous functional stilbene monomers with dialkyl amino and t-butyl ester groups in the 4,4'-position on the phenyl groups of stilbene (a), and also functional N-phenylmaleimide monomers with dimethyl amino and t-butyl ester group in the 4-position on the benzene ring of maleimide (b). We are preparing a group of novel rigid polyelectrolytes with tunable charges and charge densities by using different combinations of the donor-acceptor comonomer pairs. One of the polyanion precursors we have prepared is poly(di-t-butyl-trans-4,4'-stilbenedicarboxylate-co-t- butyl-4-maleimidobenzoate) (c). Deprotection of the t-butyl groups and neutralization of the resulted polyacid led to the all anionic polyelectrolyte (d).
Reference:
1. Turner, S. R.; Li, Y, "Free Radical Copolymerization of Methyl Substituted Stilbenes with Maleic Anhydride," European Polymer Journal, submitted.