Reports: G1
46714-G1 Remote Functionalization Reactions Catalyzed by Multinuclear Complexes
Introduction: The functionalization of C–H bonds that are remote from an activating group is a challenge that remains to be solved. Most methods for functionalizing unreactive C–H bonds are chelate-directed, and are efficient only well when the C–H bond to be transformed is in close proximity to a directing group. Developing new methods that are not limited by this distance constraint is difficult, but important for the chemical and petroleum industries because the selective conversion of hydrocarbon molecules into useful feedstocks could be achieved. For these studies, triiron basic carboxylates (TBCs) were tested for their ability to undergo directed oxidation reactions. The TBCs catalysts are attractive because many derivatives are known and these complexes are inexpensive to prepare.
Studies Conducted: During the previous grant period, we reported investigations into using TBCs in epoxidation and oxidation reactions. As described in the 2008 report, this project was terminated because low levels of conversion and regioselectivity were observed in the oxidation of organic substrates with a variety of catalysts. During the first year of the grant period, greater than 85% of the resources were spent. Resources remaining for the current grant period were used to support an ongoing project in the laboratory that is described below. Work in this area will be submitted for publication in the near future and the ACS-PRF will be acknowledged for its generous support in this publication.
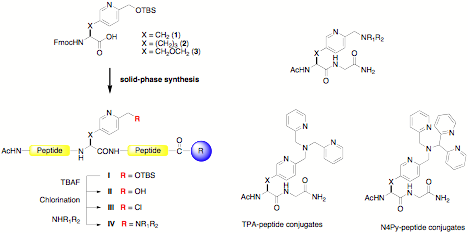
Project 1: Divergent Method for Incorporating Oxidation Catalysts into Peptide Frameworks. A divergent method for incorporating polypyridyl ligands into peptides will be reported (Figure 1). Three N-Fmoc unnatural amino acids (1-3) that contain varying linkers between the a-carbon and a 2-(hydroxymethyl)pyridyl group were synthesized in enantioenriched form. These amino acids were used as anchors for incorporating multidentate ligands onto a peptide chain in a site-specific fashion. Multiple peptide-ligand conjugates were synthesized from single precursors, by solution- or solid-phase methods. Peptides containing more than one metal-binding unit can be produced by this method. Ligands constructed by this method include tetra- and pentadentate ligands such as TPA or N4Py whose FeII complexes oxidize organic substrates including the strong C–H bonds found in hydrocarbons.1-5 In the future, such peptides may be used to create catalysts or artificial oxygenases for selective oxidation reactions and in the conversion of petroleum products into useful feedstocks.
Figure 1. Divergent Synthesis of Peptide-Ligand Conjugates
References:
(1) Osako, T.; Karlin, K. D.; Itoh, S. Carbon-Halogen Bond Activation Mechanism by Copper(I) Complexes of (2-Pyridyl)alkylamine Ligands. Inorg. Chem. 2005, 44, 410-415.
(2) Maiti, D.; Fry, H. C.; Woertink, J. S.; Vance, M. A.; Solomon, E. I.; Karlin, K. D. A 1:1 Copper-Dioxygen Adduct is an End-on Bound Superoxo Copper(II) Complex which Undergoes Oxygenation Reactions with Phenols. J. Am. Chem. Soc. 2007, 129, 264-265.
(3) van den Berg, T. A.; de Boer, J. W.; Browne, W. R.; Roelfes, G.; Feringa, B. L. Enhanced selectivity in non-heme iron catalyzed oxidation of alkanes with peracids: evidence for involvement of Fe(IV):O species. Chem. Commun. 2004, 2550-2551.
(4) Klopstra, M.; Hage, R.; Kellogg, R. M.; Feringa, B. L. Non-heme iron catalysts for the benzylic oxidation: a parallel ligand screening approach. Tetrahedron Lett. 2003, 44, 4581-4584.
(5) Nam, W. High-Valent Iron(IV)-Oxo Complexes of Heme and Non-Heme Ligands in Oxygenation Reactions. Acc. Chem. Res. 2007, 40, 522-531.