Reports: G7
46749-G7 Novel Routes to Well-Defined Conjugated Polymers and Their Block Copolymers
During the course of our research on the synthesis of conjugated polymers, we discovered a unique feature of polymers prepared from bicyclic dienes (Scheme 1). As a result of 1,4-polymerization of these monomers, a new double bond is created in the polymer backbone as a part of the bicyclic skeleton. Thus formed repeat units can undergo retro Diels-Alder (DA) reaction, or cycloreversion, when heated to certain temperatures. In the case of 1,2-dimethylenenorbornane, retro DA reaction is accompanied by ethylene release, which results in a new repeat unit structure. Such thermally triggered transformation can induce dramatic changes in polymer properties and were exploited for the preparation of advanced nanostructured materials.
Scheme 1
Three different monomers with norbornane skeletons containing exo diene groups were synthesized (Scheme 1). Free-radical polymerization of these monomers proceeded mostly by 1,4-addition, as evidenced by 1H and 13C NMR analyses. Living radical polymerization of monomers 1-3 was carried out with a universal nitroxide initiator, 2,2,5-trimethyl-3-(1-phenylethoxy)-4-phenyl-3-azahexane (TMPEPA), as this was the only documented method for controlled polymerization of dienes, such as butadiene and isoprene. When polymerizations were conducted at a typical for isoprene T = 125 °C, only polymers with broad molecular weight distributions could be obtained. However, at a lower temperature of 110 °C, we were able to produce polymers with low polydispersities for all three monomers (Table 1). A detailed kitenic analysis of nitroxide-mediated polymerization of 1 confirmed the living nature of the polymerization. Block copolymers of 1 and methyl acrylate (MA) were synthesized by sequential polymerization of each monomer. SEC analysis confirmed efficient re-initiation and the formation of well-defined block copolymers.
Scheme 2
Table 1. Nitroxide-Mediated Polymerization of Bicyclic Dienes a
|
Monomer |
Time (h) |
Conversion (%) |
Mn b (kg/mol) |
Mw/Mnb
|
|
1 |
28 |
68 |
(17.3) |
1.18 |
|
1c |
19 |
67 |
(13.5) |
1.40 |
|
2d |
10 |
70 |
19.6 |
1.20 |
|
3 |
26 |
72 |
11.5 |
1.10 |
a T = 110 °C, neat monomer; TMPEPA was used as an initiator; [M]:[TMPEPA] = 150:1. b Measured by SEC with polystyrene calibration. The numbers in parentheses were obtained from SEC with a light scattering detector. c T = 125 °C. d Conducted in 50 wt% toluene solution.
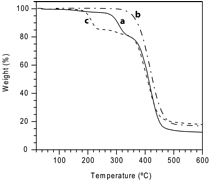
All three polymers decomposed upon heating to 360 °C. Poly(1) and poly(3) exhibited a separate weight loss step prior to degradation (Figure 1), which was attributed to retro Diels-Alder reaction taking place with a concurrent release of ethylene (Scheme 1). Weight loss of approximately 20% was observed for both polymers, in good agreement with the weight percent of ethylene in polymer repeat units. The onset transition temperatures (TDA) for poly(1) and poly(3) were 280 °C and 180 °C, respectively. A much lower transition temperature for poly(3) is driven by the formation of aromatic furan rings as the product, while the absence of such transition in poly(2) is consistent with its inability to undergo retro Diels-Alder reaction.
Figure 1. Thermogravimetric analysis (TGA) of (a) poly(1), (b) poly(2), and (c) poly(3).
Polymers of 1 and 3 were heated above TDA but below their decomposition temperatures and then cooled down to room temperature. The product of such thermal treatment of poly(3) was soluble in common organic solvents and thus could be analyzed by 1H NMR. A new strong signal at 7.2 ppm was observed in the NMR spectrum, confirming the formation of furan rings. On the hand, the product of thermal treatment of poly(1) was completely insoluble in organic solvents, suggesting the formation of cross-linked structures. We attributed this behavior to the dimerization of cyclopentadiene groups formed as a result of retro Diels-Alder reaction of poly(1). We also carried out the thermal treatment of poly(1) under vacuum, since the retro Diels-Alder reaction can be driven by removing ethylene side product. Under these conditions, poly(1) could be cross-linked at temperatures as low as 245 °C.
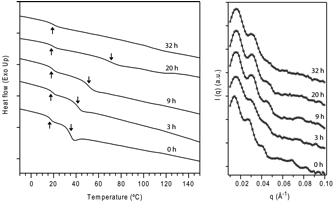
Thermal cross-linking of poly(1) was exploited for the fixation of nanostructures formed by block copolymer phase separation. Block copolymers of 1 and methyl acrylate (BDA) were annealed at 180 °C for 24 hours. At this temperature, polymers were stable and did not undergo crosslinking, which was confirmed by GPC analysis. Block copolymer BDA, with a cylindrical morphology, was then heated in a vacuum oven at 245 °C in order to promote retro Diels-Alder reaction and subsequent cross-linking. Differential scanning calorimetry (DSC) was used to monitor the formation of a cross-linked network during the thermal treatment (Figure 2). The original DSC curve (t = 0 h) shows two glass transitions at 35 °C and 18 °C, corresponding to poly(1) and poly(MA) blocks in the copolymer, respectively. The presence of two separate Tg suggests phase separation, which is in agreement with small-angle x-ray scattering (SAXS) results. After annealing at 245 °C, Tg corresponding to poly(1) block slowly increases and after 32 hours, no Tg could be observed. On the other hand, Tg corresponding to poly(methyl acrylate) block remains constant at 18-20 °C. These results clearly indicate that cross-linking is taking place in poly(1) domains, which ultimately leads to the formation of very rigid networks. At the same time, poly(MA) domains remain intact during this process.
Figure 2. DSC (left) and SAXS (right) characterization of BDA block copolymer after annealing at 245 °C. Up and down arrows indicate glass transitions of poly(MA) and poly(1) domains, respectively.
SAXS analysis confirmed the retention of formed microstructures upon crosslinking (Figure 2). The primary scattering peak and higher order reflections ( 3q0 and 7q0) were clearly distinguishable even for polymer samples annealed at 245 °C for 32 h, which had lost 50% of ethylene (TGA) and was fully cross-linked (DSC and swelling studies). While the domain size decreased slightly from 44 to 40 nm, the symmetry of the cross-linked microstructures remained the same. These findings have been submitted for publication.