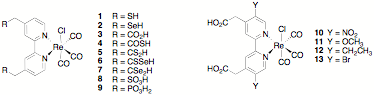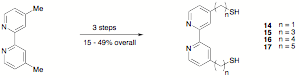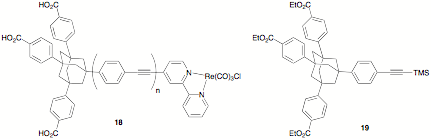Reports: B6
45966-B6 Dependence of Rates of Interfacial Electron Transfer on Anchoring Group Structure and Dye MLCT State Energy
Because there has been no systematic study of the effects on ET rate of the identity/properties of the anchoring group or of the electronic coupling between the adsorbate and the semiconductor (through matching of the energy levels of the dye to that of the semiconductor conduction band), the proposed research entails the systematic design, synthesis, and photoinduced interfacial ET studies of a series of chlorotricarbonylrhenium bipyridine complexes substituted on the bipyridine ligand with a variety of anchoring groups (complexes 1 – 9), substituents that modulate the energy levels of the ligand p-molecular orbitals (molecules 10 – 13), or that vary the distance between the dye and the nanoparticle surface (compounds 14 – 18).
In the first year of the grant, we prepared 1, 3, and 9; and in collaboration with Professors Tianquan Lian and Keiji Morokuma at Emory University, we published femtosecond IR and computational studies on their interfacial ET behavior. In addition, we also completed the synthesis of Re complex 8 and of the bipyridine ligands in compounds 4 and 5. This year (year 2 of the grant), we began to study the ET behavior of these latter complexes. Femtosecond IR studies with 8 have been hindered by difficulties getting this complex to bind to any nanoparticles, and the bipyridine ligands in 4 and 5 have been found to decompose under all conditions with which we have attempted to convert them to their chlororhenium tricarbonyl complexes.
Because of our difficulties with these compounds, we abandoned the preparation complexes 6 and 7; and we have focused on the successful synthesis of 14 – 17 and their chlororhenium tricarbonyl complexes. We have published this synthetic work, and the ET behavior of the chlororhenium tricarbonyl complexes of these ligands will be studied for comparison to our previously published results on analogs with carboxylate anchoring groups.
However, because the tethers in both the thiol complexes 14 – 17 and their carboxylate analogs are flexible, leading to ambiguity in the distance between the rhenium complex and the nanoparticle surface, we have also begun work to prepare complexes of the type 18. These molecules have a tripod anchoring group to position the phenylene-ethynylene bridge perpendicular to the nanoparticle surface, allowing for an accurate determination of distance between the surface and the rhenium center. At this point, we have prepared the precursor 19,1 as well as 4-bromobipyridine;2 and to obtain 18 with n = 1, we only need to desilylate 19, couple it to the 4-bromobipyridine, hydrolyze the ethyl esters, and complex chlororhenium tricarbonyl to the resulting molecule.
1 Galoppini, E.; Guo, W.; Zhang, W.; Hoertz, P. G.; Qu, P.; Meyer, G. J. J. Am. Chem. Soc. 2002, 124, 7801–7811. Guo, W.; Galoppini, E.; Rydja, G.; Pardi, G. Tetrahedron Lett. 2000, 41, 7419–7421.
2 Mizuno, T.; Takeuchi, M.; Hamachi, I.; Nakashima, K.; Shinkai, S. J. Chem. Soc., Perkin Trans. 2, 1998, 2281–2288.