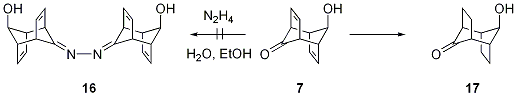Reports: GB4
45924-GB4 Synthesis and Physicochemical Investigation of Hydrofluorocarbons with Extended pi-Stacked Arene Units
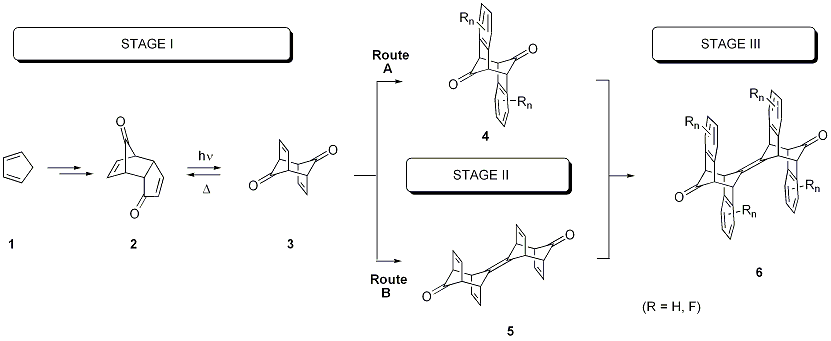
The project is directed toward the synthesis, characterization and physicochemical investigation of fluorinated, extended p-stacked arenes of type 6 along a general three-step sequence.
Figure 1: General Synthetic Scheme
During the second year we concentrated on the complete formal benzannulation of dienedione 3 toward the important intermediate 4. Furthermore, we studied the olefination of the carbonyl functionalities of cage compound 4.
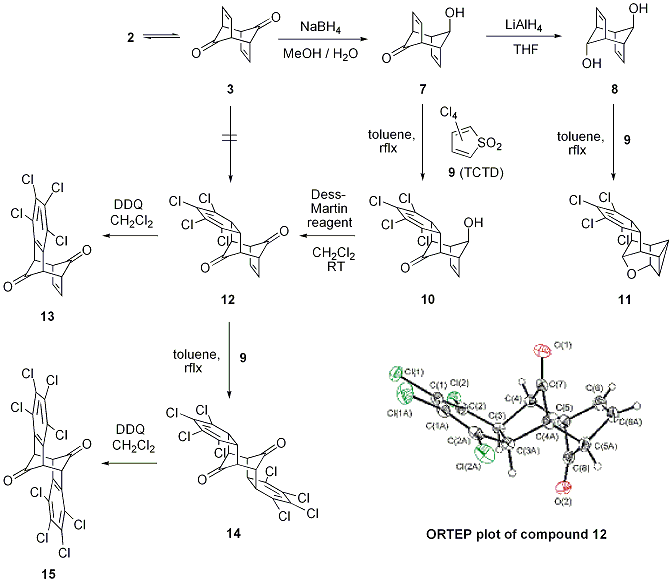
Figure 2: Diels-Alder Routes to iso-Indenone Derivatives and ORTEP plot of 12 >
Dienedione 3 is a thermally sensitive compound as it easily isomerizes back to polycycle 2. Therefore, any thermally initiated Diels-Alder reaction of substrate 3 failed and yielded complex mixtures whereas hydroxyketone 7 and diol 8 furnished the mono-adduct 10 and the transannular product 11, respectively (grant period: year 1).
Stage 2 Route A:
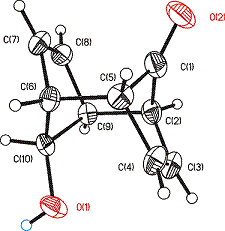
- During the second year of the grant period we were able to obtain crystal structures of dienedione 3, hydroxyketone 7 and diol 8: All three compounds show expected bond lengths and angles; the packing of the alcohols 3 and 7 display networks of hydrogen-bonding.
Figure 3: ORTEP plots of compound 7 (3, 8 are published)
- Despite many attempts to obtain a twofold Diels-Alder adduct of hydroxyketone 7, i.e., the conversion of adduct 10, we could not isolate any defined products. In refluxing xylenes compound 10 and tetrachlorothiophene (9) were quantitatively converted (no olefinic protons observable) but poor solubility of the crude product prevented the isolation and characterization of a potential bis-adduct.
- Oxidation of 10 with the Dess-Martin reagent furnished the formal twofold Diels-Alder adduct 12 of dienedione 3 within minutes. We confirmed the structure of the thermally stable polycycle 12 by X-ray crystallography. 12 was then oxidized with DDQ under mild conditions (room temperature) to the monobenzo derivative 13. The reaction is rather slow but any thermal activation would lead to a rearrangement of the product, since compound 13 contains the thermally labile subunit of dienedione 3.
- The second benzannulation of 12 with an excess of TCTD (9) provided the bis-adduct in rather low yields. Nevertheless, we could already demonstrate the oxidation of 14 to the octachloro-iso-indenone dimer 15, thus completing the synthesis of a stage 2 intermediate.
Stage 2 Route B:
· We have also attempted to couple the hydroxyketone 7 along olefination protocols to yield compounds of type 5. Unfortunately, Wittig-or McMurry type reactions furnished only complex product mixtures. Our efforts to convert starting material 7 along the Barton twofold extrusion route did not even deliver the bis-imine intermediate 16 but furnished the hydrogenated species 17, thus implying the formation of diimide (N2H2). Control experiments under strict exclusion of oxygen prevented this known side reaction but did not convert the starting material over a longer period of time either.
Figure 4: Barton Twofold Extrusion Sequence
In conclusion, we have succeeded in synthesizing two benzannulated dienedione derivatives, i.e., the monobenzo compound 13 and the formal octachloro-iso-indenone dimer. We are currently investigating the use of fluorinated building blocks (tetrafluoro-o-quinoids) at various stages of the sequence to ultimately gain access to targets of type 6.