Reports: GB6
47944-GB6 Determination of the Aggregation Propensity of Small to Large Chromophores of Asphaltene Using an Excited-State Absorption Correlation Spectroscopy Microscope
The primary goal of this research project is to develop new methodologies to study the structure and energy levels of a component of crude oil, asphaltene, over a large range of concentrations. This work is of fundamental importance because there does not currently exist a single experimental technique that can measure both the degree of aggregation and molecular properties of the aggregation such as molecular weight, dynamics etc simultaneously over a large concentration regime. The approach taken here was to find excited-state absorption transitions outside the linear absorption band that could be monitored as the concentration was increased. This is in stark contrast to previous methods such as fluorescence techniques that can only be applied through the visible and suffer from re-absorption as the concentration gets too large. Additionally, by monitoring absorption change as opposed to phosphorescence the experimental technique would be applicable to not only room temperature, but elevated temperatures as well.
The first step of this research was determining the excited-state absorption bands that are unique to the different sized chromophores of asphaltene. The excited-state absorption properties of asphaltene have not been previously examined, but there were two reasons to believe they existed. First of all, while the molecular structure of asphaltene is not exactly known, it is known to consist of fused aromatic rings and is similar to polycyclic aromatic hydrocarbons. Even the most basic polycyclic aromatic hydrocarbons, such as pyrene, have excited-states that have been characterized extensively. Second, and the most compelling reason, is that experimental evidence already exists that suggests excited-state absorption has been measured in asphaltenes in the visible spectrum using a common nonlinear optical technique called Z-scan. In this experiment a sample is scanned through the focal region of a laser beam, thus accurately varying the irradiance. In the summer of 2008 we attempted to confirm and expand on the previous reported results using the Z-scan technique on a UG8 asphaltene sample. While we were able to get some data this result was very inconsistent and the decision was made to pursue a non-degenerate technique to search for excited-state transitions.
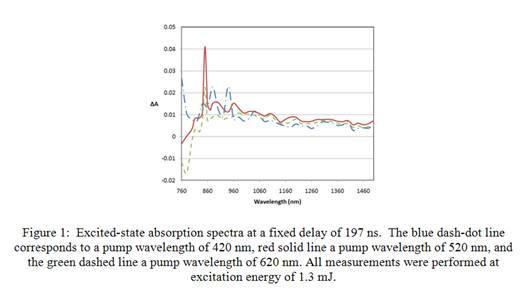
A nanosecond flash photolysis system was purchased in order to perform non-degenerate measurements to allow for a much broader search for excited-state levels as well recording the kinetics of these states. Not only have we found a broad excited-state band and characterized it's kinetics we have used several pump wavelengths to determine the dependence of this absorption band on excitation wavelength. Figure 1 shows the measured change in absorption for pump wavelengths of 420, 520, and 620 nm at a fixed delay between the pump and probe of 197 nanoseconds, and a constant energy of 1.3 mJ. A signal has also been successfully measured at excitation wavelengths out to 700 nm. This data shows that it is possible to obtain excited-state absorption for pump wavelengths above 550 nm, which is the maximum wavelength for fluorescence measurements. The most striking feature of the transient absorption spectra is how similar they are for a great range of pump wavelengths. The range, in which the spectra differ, from 760 nm to 960 nm, has a significant amount of linear absorption and therefore has the largest error bars (not shown for clarity).
My students and I have benefited in several aspects due to this grant. The chance to research and learn about issues confronted in the petroleum field that might be solved with nonlinear optical techniques was the first opportunity presented to me. After discussing with a colleague, Dr. Sudipa Kirtley, in my department at Rose-Hulman about her research in determining chemical composition of petroleum compounds I felt that some of the techniques in nonlinear optics would be useful. Dr. Kirtley put me in contact with Dr. Oliver Mullins who is a scientific advisor for Schlumberger-Doll primarily in charge of the properties of asphaltene. After having several conversations with Dr. Mullins I developed the proposal that was ultimately funded by ACS. Being awarded this proposal has meant a great deal to several aspects of my career. Shortly after being awarded this proposal Rose-Hulman offered me an additional $52,000 in internal funding to go towards the purchase of a flash photolysis system that has been primarily used on this ACS grant ($15,000 from my ACS grant went towards this piece of equipment for a total of $67,000). I have had three students work on this project for paid summer internships and two other students have contributed on various aspects during the academic year. All the students involved have truly enjoyed and have taken pride in working on this project. We recently started a collaboration with Professor Joseph West from Indiana State University, which is also located in Terre Haute IN, to measure temperature dependent optical properties of asphaltene. My two summer students really made this project move forward by working with one of Dr. West's students to integrate his temperature controlled cuvette holder and chiller system into the flash photolysis system. Not only did the three students get very nice and consistent data they have worked out diffusion based quenching model that exactly explains the data. The students are currently collaborating on writing the paper.