Reports: AC6
46671-AC6 Interactions Between Small Hydrocarbons and Water: Clathrate Formation and Inhibition
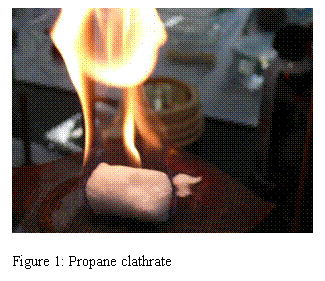
The aim of this project is to determine the interactions involved in the nucleation of clathrates: On the molecular level, these substances consist of a guest molecule, often a hydrophobic one, surrounded by a cage of water molecules. The hydrophobic molecule concentration in the clathrates is typically on the order of 105 times larger than the corresponding solubility in water. One fascinating result is that the solid looks like ice, but it can support a flame ( REF _Ref241993634 \h Figure 1). While burning snow is a novelty, the fundamental question is: How does this huge change in concentration occur? Previous experimental attempts to answer this question have mainly probed kinetics and thermodynamics associated with the growing clathrate crystal well after the nucleation process. Hence information about nucleation relied solely on theoretical models and calculations with scarce experimental data for verification. The goal of this project is to fill the gap in experimental data.
|
Figure 1: Propane clathrate
|
 Our approach to this problem is to isolate
water and the guest molecule, mainly hydrocarbons, in a solvent where water is
sufficiently dispersed that it can be probed with infrared spectroscopy. Our
initial efforts in this direction concentrated on supercritical rare gases
because they are transparent throughout the infrared. We started with argon. However,
water is not very soluble in argon. This is likely due to argon's low
polarizability. So the main result of this approach was successful design and
deployment of a high pressure optical cell.
Our approach to this problem is to isolate
water and the guest molecule, mainly hydrocarbons, in a solvent where water is
sufficiently dispersed that it can be probed with infrared spectroscopy. Our
initial efforts in this direction concentrated on supercritical rare gases
because they are transparent throughout the infrared. We started with argon. However,
water is not very soluble in argon. This is likely due to argon's low
polarizability. So the main result of this approach was successful design and
deployment of a high pressure optical cell.
The optical aperture of the cell is 20 mm and can be equipped with sapphire, CaF2, or IR quartz windows depending on the spectroscopic and physical requirements of the experiment. It has been successfully used to 100 atm. sufficiently high for all clathrates of interest.
To probe the initial interactions, we thus turned to the original solvent proposed: carbon tetrachloride. We have previously found that water exists in carbon tetrachloride as monomers. Further we modeled the dynamics of room temperature water. Carbon tetrachloride thus represents an excellent room temperature matrix isolation medium for water. The model of the dynamics is invaluable because it enables deconvolution of spectra with added solutes from that of residual water monomers to reveal the interaction dynamics between water and the solute. We have applied this to the case of small inorganic salt solutes and find that salts enter the hydrophobic phase as ion-paired clusters and water associates with the cluster primarily via interaction between the water lone pair and the cation of the salt. The anion plays an important role in polarizing the water molecule. For the combination of a small cation and large polarizable anion, the result is formation of water-water hydrogen bonds. This is a much richer picture than the expected one of an ion surrounded by an intact hydration shell.
With the detailed model for water and water interaction with ionic solutes, we then applied the carbon tetrachloride system to study the water-propane interaction. Neat propane in carbon tetrachloride ( REF _Ref241996205 \h Figure 2A) shows five infrared active modes in the C-H stretch region: symmetry reduces the eight CH stretches to only five active modes. Adding water ( REF _Ref241996205 \h Figure 2B) removes some of the degeneracy resulting in seven discernable peaks. The eighth is weak due to the near equivalence of the two terminal CH3 groups. The resonances that change significantly from propane in dry carbon tetrachloride and that with added water are the two methylene stretches. Preliminary analysis of the water portion of the spectrum suggests that the interaction is between the electropositive hydrogen atoms of the methylene group and the oxygen lone pair of one water molecule. There are at least two water molecules in the hydration shell of the propane. This is consistent with the salt results which show that water-water hydrogen bonding is best promoted by a gentle interaction between the lone pair and the solute ( REF _Ref242002075 \h Figure 3). These results are being written up for publication.
Figure 2: Infrared spectrum of (A) propane in dry carbon tetrachloride (B) propane:water mole ratio about 1:18
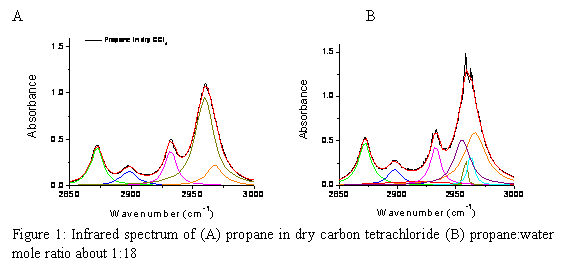
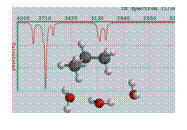
Figure 3: Cartoon of water clustered around propane. The initial interaction appears to be between the methylene hydrogen atoms and the lone pair of water.
The water-carbon tetrachloride system can be used in the high pressure cell to probe the interactions as the pressure is increased. We have preliminary data that suggests (a) the water spectrum changes little with pressure, (b) the propane spectrum changes little with pressure, but (c) the mixed system spectrum changes significantly with pressure. Further work is needed to unravel the information from these spectra.
In summary, the water-carbon tetrachloride system has proven to be an excellent, near-room-temperature matrix-isolation system for probing interactions between water and various solutes including hydrocarbons typically found in clathrates. In the next year, we will focus on interpreting the spectral changes with temperature as well as changes with both clathrate promoters and clathrate inhibitors.




