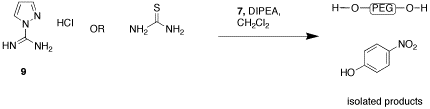Reports: GB1
47674-GB1 A New Solution Phase Protecting Group Strategy for Alkyl Guanidines
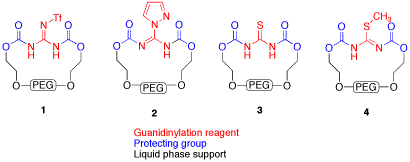
This project seeks to develop a novel protecting group strategy for the synthesis of alkylguanidines. Alkylguanidines are very basic (pKa = 13) leading to protonation and high water solubility in aqueous solutions. They are traditionally protected as carbamates to decrease basicity and water solubility. Our strategy hinges on the use of a polyethylene glycol (PEG) carbamate, which not only reduces the basicity of guanidines, but the PEG acts as a liquid phase synthesis polymeric tag,[1] and has the potential to act as a intramolecular solvent in microwave reactions.[2] Towards this end, the synthesis of four guanidinylation reagents is being explored (1-4).
Figure 1. Proposed reagents for the preparation of PEG protected guanidines.
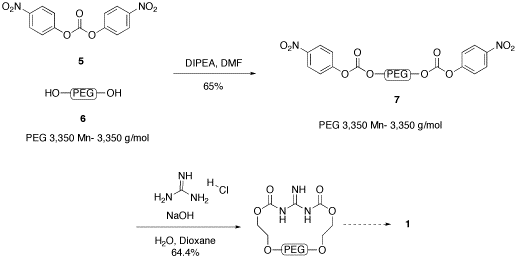
N,N'-di-PEG-N"-Triflylguanidine, 1, is being prepared in a route similar to the published preparation of N,N'-di-Boc-N"-triflylguanidine.[3] The nitrophenylcarbonate activated PEG 3350, 7, has been prepared in 65% yield from commercially available p-nitrophenylcarbonate, 5 and PEG 3350, 6 .[4] We were gratified to see that this compound is readily purified using LPOS techniques (dissolution in dichlormethane and precipitation and washing with diethyl ether). Reaction with guanidine hydrochloride produced a product that has been shown by NMR and IR to be consistent with 8. This compound was analyzed by MALDI-TOF, and found to have a mass of 3432 (C153H304N3O78, exact mass 3431.9914), with decreasing units of m/z = 44.[5] This is consistent with the desired product, with one PEG attached to the guanidine in a bidentate fashion. Further NMR work will be needed to determine definitively that the single PEG is in fact attached at both ends, as shown. Conversion of 8 to the desired guanylation reagent 1 will be carried out during the fall of 2009 by the PI, as the students who have worked on this reagent have moved on. Jennifer Yox has graduated and Nick Kelley has moved on to other interests in the Biology department.
Figure 2. Preparation of N,N'-di-PEG-N"-Triflylguanidine.
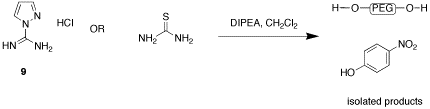
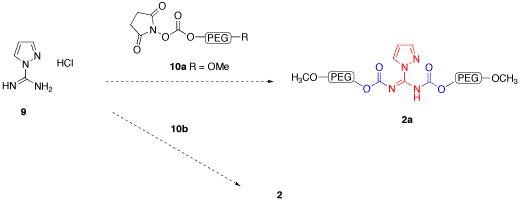
Synthesis of 1-H-pyrazole-1-N,N'-bis-PEG-carboxamidine, 2, and N,N'-bis-PEG-thiourea, 3, has been more challenging. Reaction of thiourea or 1-H-guanylpyrazolecarboxamidine hydrochloride, 9, with the 7 did not produce the desired products, with only PEG and nitrophenol being isolated (Figure 3). We are currently examining using succimidyl carbonate 10 and other activated PEGs as an alternative (Figure 4). We are also preparing an activated PEG3350 methyl ether, 10b, which will allow us to more clearly see that the PEG is coupling to both of the carboxamidine nitrogens. We also hope that the terminal methyl groups will give us another group that can be used to monitor proton integration ratios by NMR. Katie Strong has been working on this aspect of the project since May of 2008, and will continue working on it until her graduation in May of 2010.
Figure 3.
Figure 4. Preparation of succimidylcarbamate activated PEG and PEG monomethylether.
Figure 5. Planned preparation of 1-H-pyrazole-1-N,N'-bis-PEG-carboxamidine.[6]
We are starting the second year of our PRF funding period with new excitement for the project. We have a much better understanding of the challenges of working with PEG linked molecules. We are also nearing completion of the installation of 300 MHz NMR at the University of Mary Washington, which is on loan from the U.S. Navy's Naval Surface Warfare Center, in Dahlgren VA. We have found the routine analysis of these large molecules with our 60 MHz machine to be not be very useful, and will welcome in house high field analysis of our compounds. During the spring and summer of 2009, the PI was on family medical leave, due to parental illness, slowing the pace of the undergraduate students' progress.
Our focus this fall will be on the conversion of the PEG guanidine 8, to our target compound 1, and to develop a viable route to 2. We will also begin working to develop microwave heating protocols for the preparation of these compounds. During the spring of 2010, I will be recruiting two new undergraduate research students, who likely receive summer research stipends from this grant.
[1] Wentworth, P., Jr. Recent Developments and Applications of Liquid Phase Strategies in Organic Synthesis. Trends in Biotechnology 1999, 17, 488-452.
[2] Declerck, V.; Ribire, P.; Ndellec, Y.; Allouchi, H.; Martinez, J.; Lamaty, F. A Microwave-Assisted Heck Reaction in Poly(ethylene glycol) for the Synthesis of Benzazepines. European Journal of Organic Chemistry 2007, 201-208.
[3] Baker, T.J.; Tomioka, M.; Goodman, M. Organic Syntheses; Wiley and Sons: New York, 2004; Collect Vol. X, pp. 266-273.
[4] Riley, A. M.; Laude, A. J.; Taylor, C. W.; Potter, B. V. L. Dimers of D-myo-inositol 1,4,5-triphosphate: Design, Synthesis and Interaction with Ins(1,4,5) P3 Receptors. Bioconjugate Chem. 2004, 278-289.
[5] Mass spectrometry was provided by the Washington University Mass Spectrometry Resource, a NIH Research Resource (Grant No. P41RR0954)
[6] Bernatowicz, M. S.; Wu, Y.; Matsueda, G.R.; Urethane Protected Derivatives of 1-Guanylpyrazole for the Mild and Efficient Preparation of Guanidines. Tetrahedron Letters 1993, 34, 3389-3392.