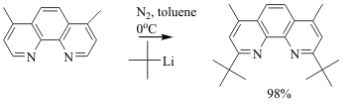Reports: B3
46743-B3 Homoleptic and Heteroleptic Copper(I) Phenanthrolines: Sterically Enhanced Excited States
Background:
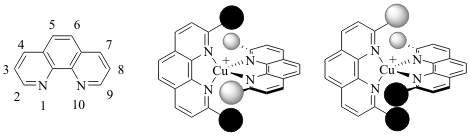
This grant year allowed for the investigation in the reactivity of methylated phenanthroline backbones as a method for creating ligands that would lead to both homoleptic and heteroleptic copper(I) complexes as represented in the Figure 1.
Figure 1: (Left) The numbering sequence for the 1,10-phenanthroline ligand. (Right) General examples of mixed phenanthroline copper complexes. The heteroleptic complex is a copper(I) with two different phenanthroline ligands. The homoleptic complex with the same ligand having differing groups in the 2 and 9 positions.
It has been previously reported that 1,10-phenanthroline reacts with a variety of lithium reagents which selectively add to the 2 and 9 positions of the ring.1 However, last year, we were surprised to report that a phenanthroline derivative, 3,4,7,8-tetramethyl-1,10-phenanthroline, had unique reactivity when the lithium reagents are either methyllithium or t-butyllithium. As shown in Figure 2, the methylated ring undergoes a nearly quantitative, single nucleophilic attack of the ring creating the mono-substituted product when the R group is -CH3 or -C(CH3)3.
Figure 2: Reaction scheme for the nucleophilic aromatic substitution on 3,4,7,8-tetramethyl-1,10-phenanthroline (tmp) with excess lithium reagent. The only product formed has the R group in the 2 position.
We began our project to study two hypotheses that supported the unique reactivity displayed by this starting material. As it is with most organic reactions, the unexpected reaction product is often attributed either to steric control of the starting material and/or reactant or to the electronics of the reaction. Our first hypothesis was that the methyl groups at the 3 and 8 positions on the starting material provided a steric encumbrance to the addition of a group as large as the t-butyl group. To test this idea, 4,7-dimethyl-1,10-phenanthroline was used as a starting material to remove the possible steric interaction at the 3 and 8 positions. Our result was that the t-butyllithium reagent not only added to the 2 and 9 positions but also reacted in a quantitative yield of one newly reported product as seen in Figure 3. This yield is a significant increase over the 30% reported from the 1,10-phenanthroline starting material which also gave a mixture of products that require column separation.1
Figure 3: Reaction scheme for the creation of the new 2,9-di-t-butyl-4,7-dimethyl-1,10-phenanthroline.
The reaction result outlined in Figure 3 suggests that methyl groups in the 4 and 7 positions activate the 2 and 9 positions toward nucleophilic attack. This is being studied using other lithium reagents to confirm this finding is not unique to the t-butyllithum reagent. This reaction is of significant note as the recently reported copper(I) complex with 2,9-di-t-butyl-1,10-phenanthroline was reported this year with the longest lived excited state lifetime yet for a copper complex.2
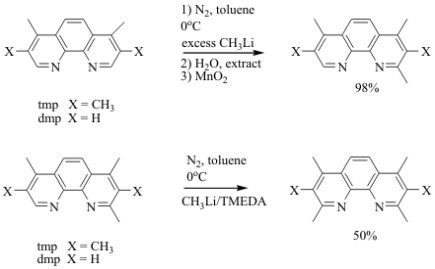
In contrast to the t-butyl group, it was unlikely that sterics of the starting material were preventing the methyllithium reagent from attacking the 2 and 9 positions of the phenanthroline ring. Therefore, our second hypothesis was that the methyl groups on the backbone of the ring were creating an electronic deactivation of the nucleophilic alpha-carbon which prevented the methyl anion from attacking twice. To test this hypothesis, we first confirmed that the 4,7-dimethyl-1,10-phenanthroline, when used as a starting material, did not create the di-substituted product with methyllithium and instead created the 2,4,7-trimethyl-1,10-phenanthroline. After isolating this mono-substituted product, it was reacted with a slurry of N,N,N',N'-tetramethylethylenediamine (TMEDA) and methyllithium. The TMEDA is known to disrupt the aggregation of the CH3Li in solution which increases the reactivity of this reagent as a nucleophile.3 As outlined in the reaction below in Figure 4, we were able to convert half of the starting material into the di-substituted product we were attempting to create.
Figure 4: Reaction scheme creating the di-substituted phenanthrolines in a step-wise process. The two products are the 2,3,4,7,8,9-hexamethyl-1,10-phenanthroline (hmp) and the 2,4,7,9-tetramethyl-1,10-phenanthroline.
Column chromatography allows for the isolation of each pure substance. Normally, these ligands are created using a multi-step, ring-closure synthesis with a 10% overall yield.4,5 This new reaction scheme not only confirms that the methylated starting material deactivated the alpha-carbon to nucleophilic attack, but it also afforded a significant improvement over previous syntheses.
In summary, we have evidence that these methylated phenanthroline backbones are deactivating the 2 and 9 positions in two ways, sterically and electronically, depending on the alkyllithium reagent that is used as the nucleophile. Future work will involve creating the copper(I) complexes and investigating the photochemistry - all of which are new additions to the field.
This grant year involved the my supervision of four undergraduates, including one SUMR student, as a part of the PRF goal to prpare students for graduate studies or emplyment. This ongoing project was a topic of student presentations at Argonne National lab in the Fall 2008, poster presentations at the Spring 2009 ACS meeting, and two on-campus presentations.
References:
1) a) Dietrich-Buchecker, C.O.; Marnot, P.A.; Sauvage, J.P. Direct Synthesis of Disubstituted Aromatic Polyimine Chelates. Tetrahedron Lett., 1982, 23, 5291. b) Pallenberg, A. J.; Koenig, K. S.; Barnhart, D. M. Synthesis and Characterization of Some Copper(I) Phenanthroline Complexes. Inorg. Chem. 1995, 34, 2833-2840.
2) Green, O; Gandhi, B.A.; Burstyn, J. N. Photophysical Characteristics and Reactivity of Bis(2,9-di-tert-butyl-1,10-phenanthroline) Copper(I) Inorg. Chem. 2009, 48, 5704–5714.
3) Stey, T.; Stalke D. In The Chemistry of Organolithium Compounds; Rappoport, Z., Marek, I., Eds.; Wiley: Chichester, 2004; pp 47-120.
4) Case, F. H.; Wesniski, H.A. The Synthesis of Polymethylquinolines and 1,10-phenanthrolines J.Hetero. Chem., 1968, 789.
5) Butt, G.; Topsom, R.D. A Simple Synthesis of Some 1,l0-Phenanthrolines J. Heterocyclic
Chem., 1981, 18, 641.