Reports: G1
48346-G1 Addition-cycloisomerization of Propargylcyanamides; Efficient Access to the 2-aminoimidazole Core
Narrative: Our research program ultimately requires synthetic chemistry to study the biological functions of structurally unique natural products with significant biomedical relevance. The synthetic methodology that was developed during this grant cycle has been extremely valuable to accomplishing these goals. This grant has helped support two graduate students (1/2 time). It has solidified this synthetic manifold as a major emphasis of our research program and will be the foundation of both students' Ph.D. dissertations. As a beginning researcher this grant has been invaluable for student support to help accelerate the research accomplishments described in this narrative.
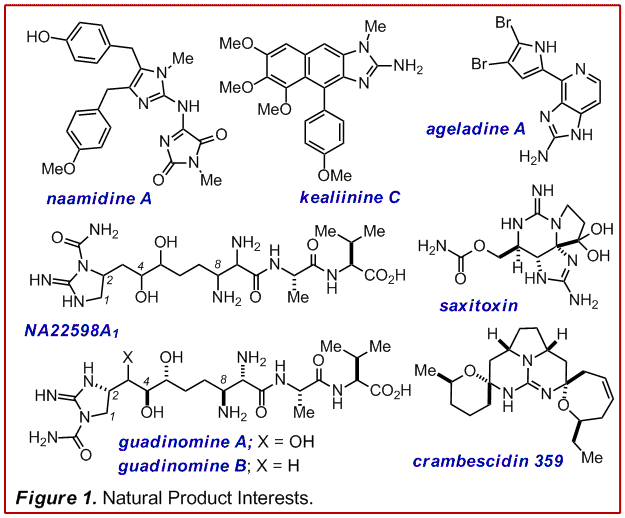 We have a continued interest in the
synthesis and biology of guanidine containing natural products, due to the
importance of this motif in the molecular recognition of carboxylate, sulfate
and phosphate groups in biological macromolecules (Curr. Bioact. Cmpds. 2009, 5, 39-78). For example we are interested in
the Leucetta alkaloids of which naamidine A and kealinine C are
representatives (Figure 1). These compounds display a diverse range of
biological activities based on a relatively conserved structural core. NA22598A1
or the guadinomines also represent an important arena for target identification
and mechanism of action studies. NA22598A1 was identified as a selective
inhibitor of anchorage independent growth (AIG), suggesting a potential lead
for small molecules that can selectively inhibit tumor metathesis. The
guadinomines were identified as agents capable of inhibiting the Type 3
Secretion System (T3SS), a transmenbrane protein complex that directs the infection
of host-cells with bacterial
We have a continued interest in the
synthesis and biology of guanidine containing natural products, due to the
importance of this motif in the molecular recognition of carboxylate, sulfate
and phosphate groups in biological macromolecules (Curr. Bioact. Cmpds. 2009, 5, 39-78). For example we are interested in
the Leucetta alkaloids of which naamidine A and kealinine C are
representatives (Figure 1). These compounds display a diverse range of
biological activities based on a relatively conserved structural core. NA22598A1
or the guadinomines also represent an important arena for target identification
and mechanism of action studies. NA22598A1 was identified as a selective
inhibitor of anchorage independent growth (AIG), suggesting a potential lead
for small molecules that can selectively inhibit tumor metathesis. The
guadinomines were identified as agents capable of inhibiting the Type 3
Secretion System (T3SS), a transmenbrane protein complex that directs the infection
of host-cells with bacterial
effector
proteins. Other marine metabolites with guanidines serve as 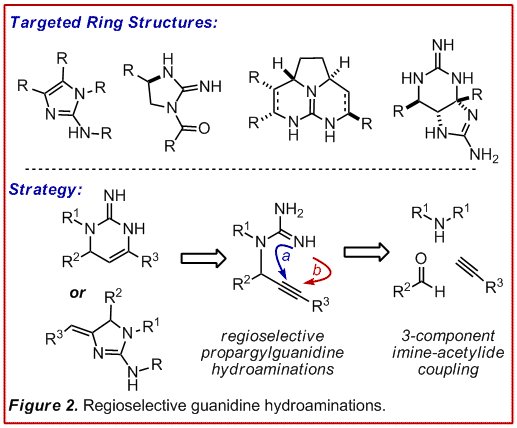 synthetic inspiration
such as saxitoxin, the crambescidins and the batzelladines.
synthetic inspiration
such as saxitoxin, the crambescidins and the batzelladines.
Synthetic platform: Given the range of structural diversity in these natural products in respect to ring size, oxidation state and substitution we aimed to develop a unified synthetic platform to access these skeletons. In pursuance of our interests, a unified approach must:
1) provide access to predictable and controlled substitution patterns in short order,
2) deliver these diverse hydrogen bond topologies, and
3) permit ring oxidation state adjustments within these cyclic or polyclic structures.
We
felt that the addition of a guanidine N-H bond across a C-C p-system
would represent a powerful tool for the preparation of these challenging
heterocyles and was the 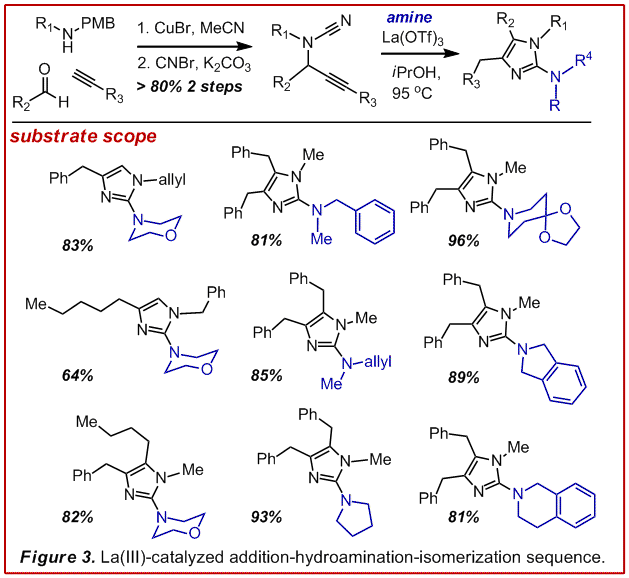 basis of our ACS-PRF proposal. The
development of amine-alkene/alkyne hydroamination chemistry has provided an
invaluable tool for the construction of nitrogen based heterocycles. The
extension of this methodology to guanidines, however, remains largely
underdeveloped. In order to manipulate the oxidation states of these
heterocyclic cores we anticipated that this is best done by reduction, as
oxidations of nitrogen rich heterocycles are usually problematic. This has led
us to study the addition of guanidine N-H bonds across alkynes, thus providing
access to cyclic structures at an elevated oxidation state (Figure 2). This
disconnection also permits the rapid
basis of our ACS-PRF proposal. The
development of amine-alkene/alkyne hydroamination chemistry has provided an
invaluable tool for the construction of nitrogen based heterocycles. The
extension of this methodology to guanidines, however, remains largely
underdeveloped. In order to manipulate the oxidation states of these
heterocyclic cores we anticipated that this is best done by reduction, as
oxidations of nitrogen rich heterocycles are usually problematic. This has led
us to study the addition of guanidine N-H bonds across alkynes, thus providing
access to cyclic structures at an elevated oxidation state (Figure 2). This
disconnection also permits the rapid 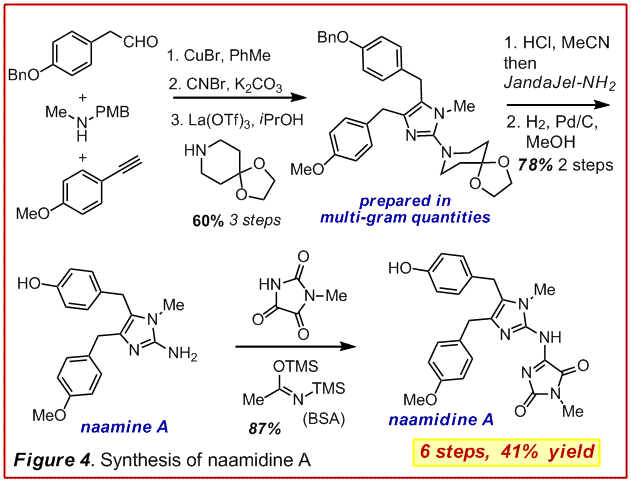 construction of diverse skeletal
precursors via an imine-acetylide 3-component coupling.
construction of diverse skeletal
precursors via an imine-acetylide 3-component coupling.
To this end we examined the utility of an addition-hydroamination-isomerization sequence (Figure 3). (Angew. Chem. Int. Ed. 2009, 48, 3116-3120). This strategy provided access to a variety of highly substituted 2-aminoimidazoles in just 3 steps and was generally high yielding. Further, the introduction of a removable R group permitted the preparation of any desired hydrogen bond donor-acceptor topology. This manifold has allowed us to execute the shortest, highest yielding and most flexible synthesis of naamidine A (Figure 4, unpublished). In short order, this technology will allow us to examine structure-activity-relationships and prepare chemical biological probes to delve into the mechanism of action for naamidine A.
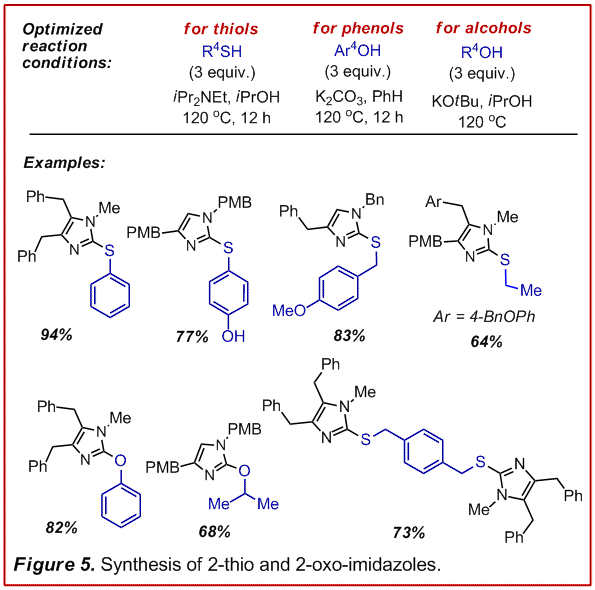 We have also been able to adapt this
reaction sequence to the addition of thiols, phenols and alcohols (Figure 5, manuscript
in preparation for J. Org. Chem.). Consistent with what we had learned from
the addition of amines to the cyanamides, this chemistry requires a fine
balance of nucleophilicity and basicity to effect addition to the cyanamide
without formation of the allenyl-cyanamide which readily decomposes. This
manifold presents an interesting polarity reversal for the preparation of these
heterocycles which are usually constructed by the addition of an intact
thiourea or urea to an electrophile. This greatly expands the scope of building
block diversity that can be used to decorate these
We have also been able to adapt this
reaction sequence to the addition of thiols, phenols and alcohols (Figure 5, manuscript
in preparation for J. Org. Chem.). Consistent with what we had learned from
the addition of amines to the cyanamides, this chemistry requires a fine
balance of nucleophilicity and basicity to effect addition to the cyanamide
without formation of the allenyl-cyanamide which readily decomposes. This
manifold presents an interesting polarity reversal for the preparation of these
heterocycles which are usually constructed by the addition of an intact
thiourea or urea to an electrophile. This greatly expands the scope of building
block diversity that can be used to decorate these 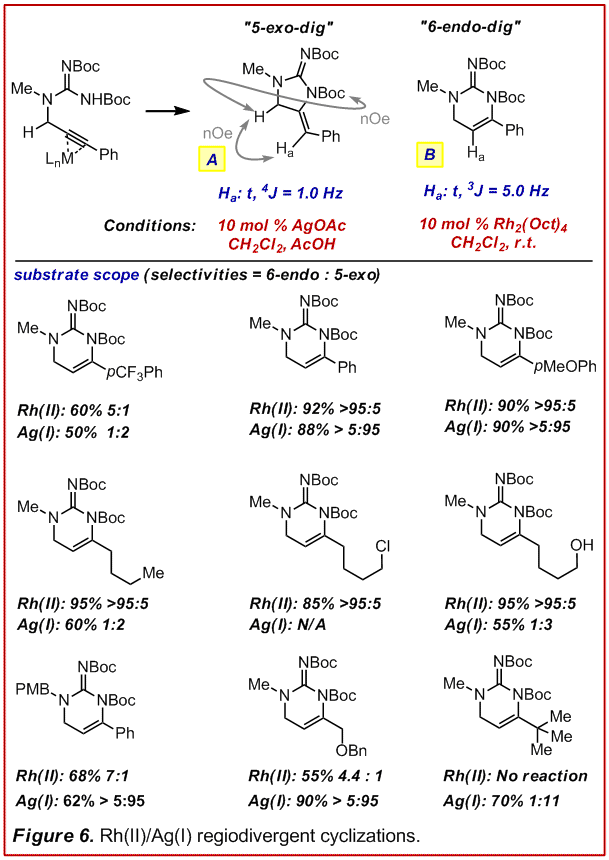 cores.
cores.
The major limitation of this methodology was that the addition of a nucleophile to the cyanamide was rate limiting and required forcing temperatures (>95 oC). Under these conditions only the stereo-electronically favored 5-exo-dig cyclization was observed followed by isomerization. Thus we set out to develop a more mild cyclization procedure that would allow us to A) selectively generate the 5-exo-dig or the 6-endo-dig product and B) maintain the fidelity of the alkene position so that it can be used for further functionalization. Under the simplistic assumption that a p-Lewis acid might trigger the hydroamination process, a series of metal salts were evaluated for their ability to affect the cyclization of an intact N,N-diboc-propargylguanidine (Figure 6, manuscript in preparation for J. Am. Chem. Soc.). In short we found that Ag(I) catalysts were optimum for the formation of the formal 5-exo-dig product. We also discovered a unique role of dirhodium carboxylates to promote the selective formation of the formal 6-endo-dig product. Of particular note is the fact that Rh(II) is highly selective for alkynes whose termini are not electronically differentiated (e.g. alkyl, see row 2). Rh(II) can also tolerate alkyl-halides which will be important for subsequent annulations etc. in the formation of more elaborate natural product skeletons.
The mechanism of Rh(II) catalysis appears to be quite complex. Both Hammett selectivity plots and competition experiments suggest that the 5-exo-dig and 6-endo-dig products arise from distinct mechanistic pathways. At this point we hypothesize that these are a partition of migratory insertion versus elecrophilic cyclization. Studies are currently ongoing to elucidate these mechanistic details. If our mechanistic hypothesis is supported, it would represent a unique manifold in hydroamination chemistry whereby product regio-selectivity can be controlled by conditions that favor a specified mechanistic pathway and is not subject to electronic effects inherent in the substrate.




