Reports: B1
45440-B1 Exploiting the Oxacalixarene Scaffold: Structural Diversity, Macrobicyclic Hosts, Multicalixarenes, and Molecular Tweezers
Research Summary September 2008 – September 2009
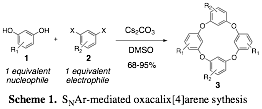
The Katz group continues to be a leader in developing methods for the synthesis of oxacalix[4]arene macrocycles by nucleophilic aromatic substitution (SNAr) reactions of meta-diphenols 1 and meta-dihalogenated aromatics 2 (Scheme 1). The operational simplicity of the procedure, coupled with the high substrate scope, have allowed us to efficiently access and explore the applications of a wide variety of complex oxacalix[4]arenes 3. These reactions are conducted under conditions of thermodynamic product control, and the high product yields obtained, in many cases over 90%, are the result of an unusually strong thermodynamic preference for the corresponding oxacalix[4]arene over all other linear and cyclic oligomeric products.
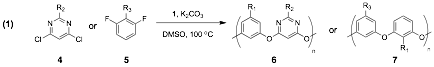
Over the past year, we have undertaken an investigation targeting the single-step formation of large-ring oxacalix[n]arenes (n>4) under conditions of kinetic product control. During this investigation we unexpectedly uncovered that certain electrophiles resist kinetic oxacalixarene formation, instead undergoing facile polymerization to rare class of polymers – poly(m-phenylene oxides) (mPOs). 1 Poly(mPOs) are potentially useful thermostable materials with properties similar to PEEK plastics, but have previously proven difficult to synthesize by SNAr or other means due to competing cyclooligomer (oxacalixarene) formation. Our recent manuscript ("Selective Synthesis of Poly(m-Phenylene Oxides) over Oxacalixarenes" Wackerly, J. W.; Meyer, J. M.; Crannell, W. C.; King, S. B.; Katz, J. L., Macromolecules 2009, in press.) details that poly(mPOs) 6 and 7 are indeed accessible in high yield by SNAr reactions through AA-BB type step-growth polymerization, by condensation of meta-diphenols 1 and meta-dihalogenated aromatics 4 or 5 (Eq 1). Mechanistic investigations have revealed that the linear (polymeric) vs. cyclic (oligomeric) product distributions are strongly dependent on the substitution pattern of the electrophilic monomer. The synthesized poly(mPOs) have high thermal and aqueous stability but are readily depolymerized in polar aprotic media. Thus, the materials have potential engineering and biomedical uses, as well as the benefit of facile post-use recycling or processing. Our future investigations of poly(mPOs) will target: 1) extending the scope of materials available through our method by exploring compatible functional groups and substitution patterns on both monomers, 2) controlling and optimizing the molecular weights of desirable polymers to increase oxidative and thermostability, and 3) exploring the effect of functional group and molecular weight alterations on the chemical and physical properties of the materials.
1 (a) Beeson, J. H.; Pecsar, R. E. "Poly-M-phenoxylene" Anal. Chem. 1969, 41, 1678-1682. (b) Cotter, R. J.; Engineering Plastics, A Handbook of Polyarylethers; Gordon and Breach: Basel, Switzerland, 1995; Chapter 1.