Reports: AC3
47426-AC3 Discovery of Molecular Oxidation Catalysts using Metallo-Enzyme Mimicry and Combinatorial Chemistry
Aims:
The aims of this project are:
(1) To develop routes to new polytopic ligands and/or ligand precursors capable of forming multi-metallic complexes combining organometallic N-heterocyclic carbene (NHC) centres and one or more 'classical' metal sites.
(2) To find methods to selectively form multimetallic complexes of the afore-mentioned ligands or ligand precursors.
(3) To use these selective methods to prepare libraries of multimetallic complexes that combine organometallic – organic substrate activating – centres with classical – dioxygen/peroxide activating – centres.
(4) To assay these libraries for efficient catalysis of selected oxidations of organic substrates.
Personnel:
A/Prof. S.B. Colbran and Prof. D.B. Hibbert are the principal investigators. SBC is involved in all aspects of the project. DBH is a chemometrician and his main role deals with the design of efficient methodologies to screen of the libraries of complexes for oxidative catalyses, which comes in the later stages of the project.
ACS-PRF funds have been provided for three researchers that have done all of the laboratory work: Dr Sang-Tae Lee has been employed as a part-time research assistant working on the synthesis since the project's inception in July 2008. Most of the results summarised below are from his work. Mr Alex McSkimming, a third year BSc student, was part funded as a summer scholar from the ACS-PRF grant, and Mr Matthew Peterson, another third year BSc student, has been funded part time from the ACS-PRF grant to prepare the mono-metal equivalents of each metal centre in the multi-metallic species, and to assay these and mixtures of these for oxidation catalyses.
Progress:
Our progress, to date, can be summarised as follows:
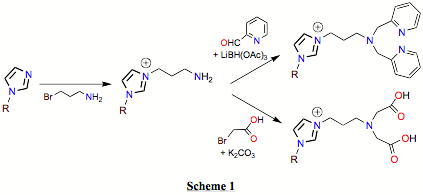
(1) After several
unfruitful mis-directions, a new simple route to imidazolium salt precursors to
NHC-ligands that are tethered to
'classical' donor groups has been found. The route is simple and has great
versatility. We find that treatment of any imidazole with 3-bromopropan-1-amine
affords the amine-tethered imidazolium salts, which are readily isolated as the
PF6– salts upon metathesis with NH4PF6
in methanol–water mixtures, see Scheme 1. The amino-tether can be then
elaborated by standard methods to build-up the classical donor groups, e.g.
reductive amination with 2- carboxaldehyde pyridine affords a pendant
bis(2-pyridylmethyl)amine (bpa)
donor and alkylation with 2-bromoacetic acid gives a pendant amino(diacetic
acid) donor, Scheme 1. Scheme 2 shows the metal complexes of the two such
ligands that our on-going studies are centred on.
(2) Selective
metalation of the various metal centres in the polytopic ligands has proved to
be problematic. For example, complex (1) in Scheme 2 is thus far limited to a RhCl(cyclooctadienyl)
organometallic centre because selective routes to other organometallic centres,
e.g. of Ru or Pd, have not yet been found. For example, palladium precursors
bind at both the NHC and the classical bpa donor domains. While not the targets of this work,
such molecules are new and of interest in themselves.
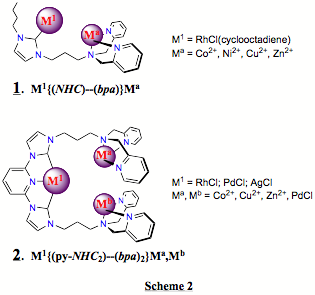
(3) Tying-up the
'classical' domain by selective metalation is relatively easy and can
facilitate subsequent selective metalation of the organometallic domain. This
seems to be the most selective route to the trimeric systems represented by complex
(2) in Scheme 2. For example,
'strong' coordination of the two bpa domains in (2) with, e.g., 'PdCl' (introduced as PdCl2(PhCN)2)
allows selective metalation of the 2,6-bis(imidazolium)pyridine domain with to
afford trimeric systems with{bis(NHC)-pyridine}RhCl, {bis(NHC)-pyridine}PdCl and {bis(NHC)-pyridine}AgCl organometallic centres. However,
prior 'weak' coordination of the classical bpa-domains may be more useful. An example: addition of
Zn(NO3)2 to the ligand precursor to (2)
affords two (--bpa)Zn2+
centres. With an excess of Zn2+ present in solution, selective metalation
of the 2,6-bis(imidazolium)pyridine centre affords the corresponding
{bis(NHC)-pyridine}RhCl and {bis(NHC)-pyridine}PdCl organometallic centres. The
zinc can then be removed by washing with excess ammonia solution giving two
metal-free bpa-pendants now able
to bind virtually any metal cation to give the classical coordination sites.
(4) Monomeric
analogues of each of the metal centres in dimers (1) and trimers (2) have been prepared. Screening of these
individually and in mixtures as catalysts for the oxidation of styrene is
underway.
Future research:
In Year 2 of this ACS-PRF funded project, we will concentrate on systems (1) and (2), and particularly on building up libraries with an extensive range of metal ions in the classical sites. These libraries will be assayed to identify catalysts for oxidations of organic substrates as outlined in the original grant application.




