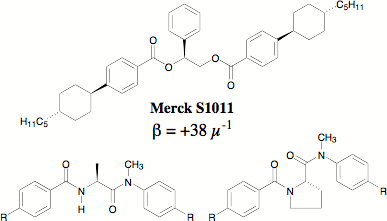Reports: GB7
46049-GB7 Design and Synthesis of Chiral Nematic Liquid Crystal Twist Agents
Twist agents are chiral compounds that are doped into the achiral nematic liquid crystal phase to induce a helical ordering and thus create a chiral nematic phase (N*), also referred to as a cholesteric phase. Chiral nematic liquid crystals (LCs) are the most commercially exploited of the LC phases and are the active organic component in the majority of LC display technologies. The figure of merit for a dopant's ability to induce helicity is helical twisting power (HTP or beta) defined as 1/p(c)(r) where p is the helical pitch in microns, c is weight percent of dopant, and r is the enantiomeric excess. Thus, to decrease helical pitch length one either adds more of the same dopant, or adds a dopant with higher HTP. The sign of beta defines the handedness of the N* helix and is related to (but not predicted by) the absolute stereoconfiguration of the enantiomeric dopant. L-Amino acids are versatile and inexpensive chiral pool reagents and have been previously incorporated into LC systems. We have completed the synthesis of four new chiral nematic twist agents derived from L-alanine and L-proline and structurally analogous to the commercial twist agent Merck S1011 as shown in Figure 1. Methylation of the aryl nitrogen of the diamides was essential for imparting solubility in the achiral nematic host E7.
Figure 1. New amino acid-based diamide dopants analogous to S1011
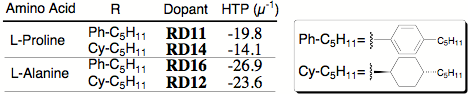
The dopants were each dissolved in E7 at three different concentrations, and HTP was determined from the slope of a plot of 1/pitch vs. concentration. Linearity of the data forced to include the origin was confirmed by linear regression (R greater than 0.9988). Table 1 shows the HTP of the new dopants.
Table 1. Helical twisting power of new dopants.
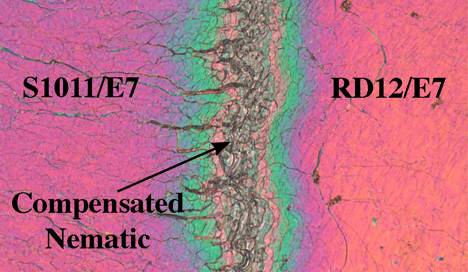
We see two prominent results: 1) Alanine-based twist agents have significantly higher HTP than proline-based dopants, and 2) Biphenyl aromatic units impart slightly higher HTP than do phenylcyclohexyl units. Interestingly, all four of the L-amino acid based twist agents induce the opposite helical sense than S1011 (despite sharing an S absolute configuration) as demonstrated by contact preparations involving S1011 and the new dopants, both dissolved in E7. When chiral nematic phases of opposite helical sense flow together, a prominent region of untwisted compensated nematic (i.e., infinite pitch) occurs at the interface (Figure 2).
Figure 2. Polarized micrograph of contact preparation between RD12 and S1011
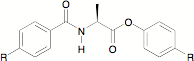
In an effort to understand the relationship between HTP and molecular shape, we are correlating calculated minimum energy conformations of new dopants with HTP. Thus, to expand our library of compounds, our next targets are the conformationally less restricted amido esters shown in Figure 3.
Figure 3. L-Alanine derived amido-ester chiral nematic twist agent
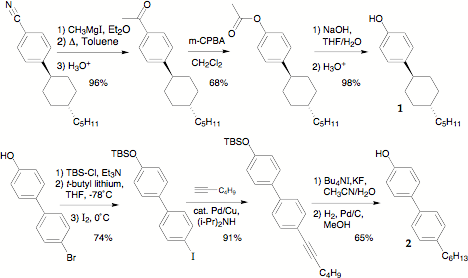
We have completed the synthesis of the key phenol intermediates as shown in Scheme 1. However, esterification with N-protected amino acids has proven problematic, with hydroysis of the phenyl ester competing with deprotection of the amine. Confirming normal reactivity of phenol 2 as well as the alkynyl precursor, we synthesized two new LCs in high yield shown in Figure 4.
Scheme 1. Synthesis of phenolic precursors to amido-ester twist agents
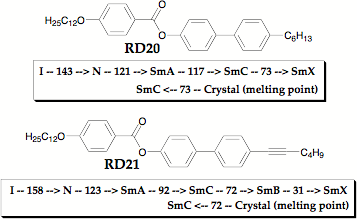
The two new LCs differ by only four hydrogens and both exhibit a number of different smectic phases. The similarity of the two mesogens allows us to directly assess the effect of the alkynyl tail relative to the alkyl tail. The phase diagrams reveal that the alkynyl tail favors untilted smectic phases as demonstrated by the wider SmA phase of RD21 as well as the occurrence of an (untilted) SmB phase below the (tilted) SmC. SmX refers to an unidentified, higher-ordered tilted smectic phase.
Figure 4. Phase diagrams of smectic liquid crystals comparing alkyl and alkynyl tails