Reports: GB1
45092-GB1 Synthesis and Transition Metal-Catalyzed [3+2] Cycloadditions of Methyleneaziridines
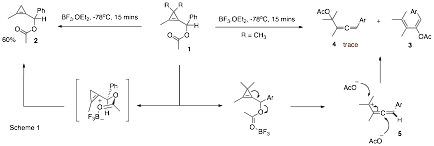
In the first year of this grant a side-project investigating the reactivity of cyclopropenyl acetates provided a new avenue of investigation. Cyclopropenyl acetates are interesting building blocks for organic synthesis due to their inherent strain and the presence of binding sites for both p-philic and s-philic Lewis acids. It was initially found that in the presence of the s-philic Lewis acids BF3.OEt2 rearrangement of cyclopropene 1 to heteroatom substituted methylenecyclopropane 2 occurred (Scheme 1). Interestingly, the presence of a gem-dimethyl group on the cyclopropene ring led to the formation of acetoxydiene 3 with high stereoselectivity. The formation of diene 3 was accompanied by trace amounts of allene 4, which lends support to the intermediacy of an allenyl cation 5.
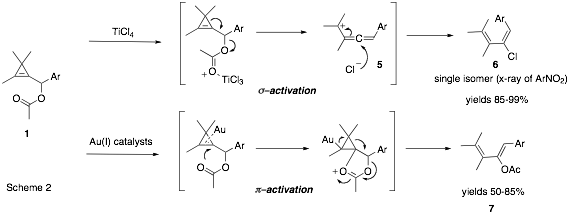
Further screening of Lewis-acids led to the discovery that TiCl4 causes cyclopropenyl acetates 1 to rearrange to (E)-chlorodienes 6. Again, this rearrangement is thought to proceed via an allenyl cation 5, which is then intercepted by chloride from the least hindered face of attack. Much of the work this year focused on optimizing the conditions for the TiCl4 mediated rearrangement and exploring substrate scope and the reaction has proved general for a range of cyclopropenes with different Ar groups. Given then both BF3.OEt2 and TiCl4 are acting as s-philic Lewis-acids activating the acetate moiety of the cyclopropene we sought to find Lewis-acids that would activate the p-bond of the cyclopropene. Towards this goal we have investigated the behavior of cyclopropenyl acetates 1 in the presence of gold(I) and gold (III) catalysts. The results of this investigation have shown that the cyclopropenes rearrange rapidly and efficiently to (Z)-acetoxydienes 7 at low temperature. This result is exciting because the selectivity is opposite to that observed for the BF3.OEt2 mediated process. A tentative mechanism for this gold-catalyzed reaction is shown in Scheme 2, but further mechanistic studies need to be carried out. The reaction has so far proven general for a range of aryl groups and we are currently preparing more examples for publication in the very near future. In conclusion, we have found a diverse set of Lewis-acid catalyzed and mediated rearrangements of cyclopropenyl acetates that allows stereoselective preparation of substituted dienes. Such dienes are important as they can be deployed in the Diels-Alder reaction and notably are difficult to prepare using existing synthetic methods.