Time for the Test!
It's a typical day in the lab, and you have two unknown samples of methane from Earth's atmosphere waiting to be analyzed. Using the procedure described previously, the methane samples have been combusted and purified to form carbon dioxide and water. Click below to analyze the carbon dioxide from one or both samples in the mass spectrometer. Then record the values for masses 44(12C162) and 45(13C162).
It's a typical day in the lab, and you have two unknown samples of methane from Earth's atmosphere waiting to be analyzed. Using the procedure described previously, the methane samples have been combusted and purified to form carbon dioxide and water. Click below to analyze the carbon dioxide from one or both samples in the mass spectrometer. Then record the values for masses 44(12C162) and 45(13C162).
The values that you recorded for mass 44 corresponds to the abundance of the 12C isotope, and mass 45 corresponds to 13C. Divide the abundance of the 13C isotope by the abundance of 12C to determine the 13C/12C ratio. to convert your ratios into delta notation, use the formula below. Rsample is the ratio you have calculated experimentally, and Rstandard is the standard 13C/12C ratio of 0.0112372, based on the standard fossil PeeDee Belemnite.
δ13C = (Rsample - Rstandard⁄Rstandard) • 1000
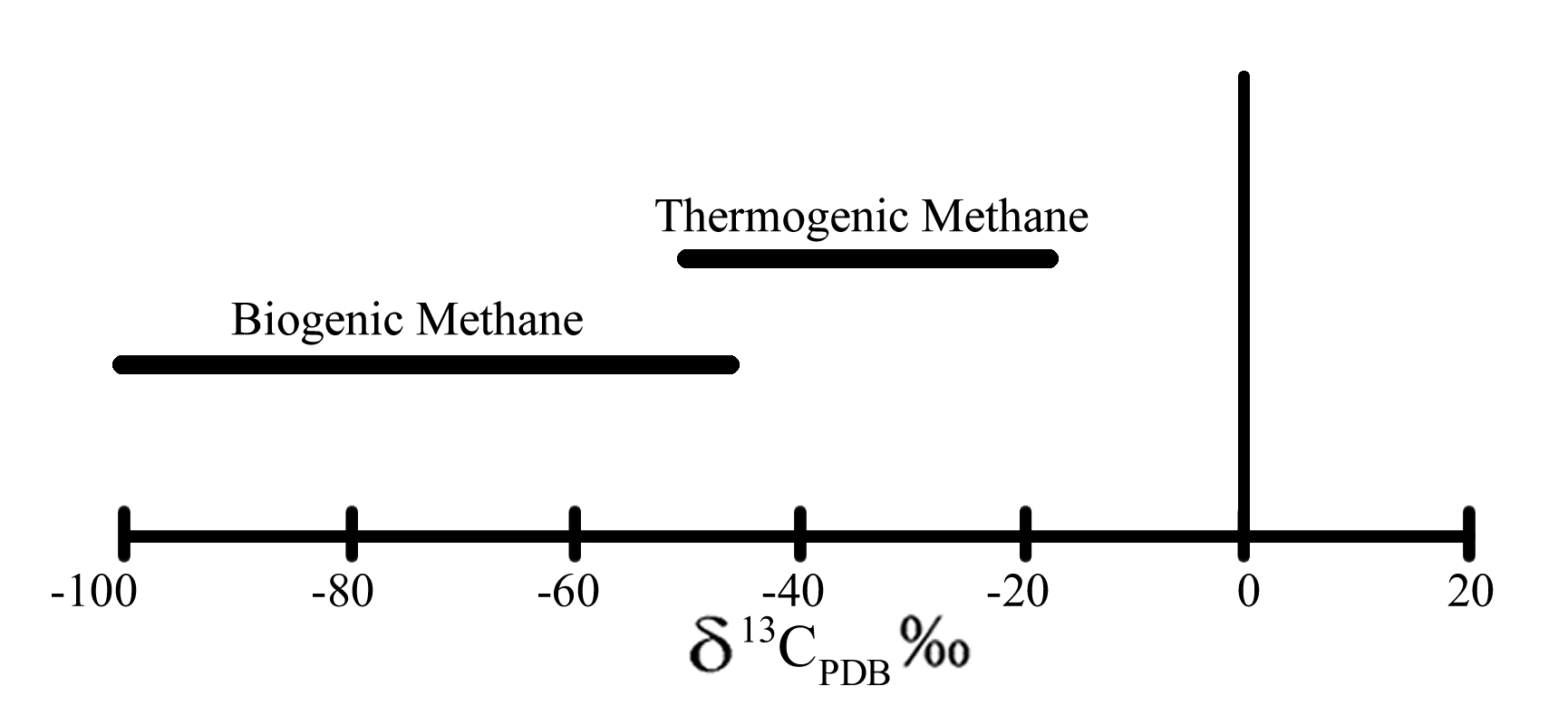
Using your calculations and the graph to the right, see if you can determine whether your methane sample is most likely thermogenic or biogenic. What are some possible sources the methane could have come from?
Next Page